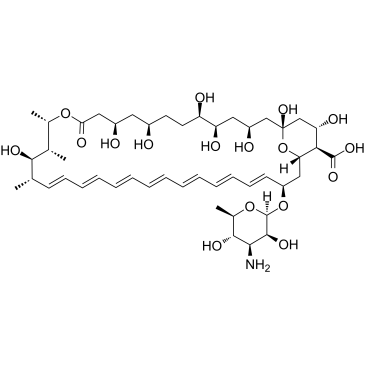
Amphotericin B
CAS No. 1397-89-3
Amphotericin B( Abelcet | AmBisome | Amphocin | Ampho-Moronal | Amphozone )
Catalog No. M11661 CAS No. 1397-89-3
Amphotericin B is a Lipid-based Polyene Antifungal and Polyene Antifungal. The chemical classification of amphotericin b is Polyenes.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 30 | In Stock |


|
| 100MG | 41 | In Stock |


|
| 500MG | 68 | In Stock |


|
| 1G | 88 | In Stock |


|
Biological Information
-
Product NameAmphotericin B
-
NoteResearch use only, not for human use.
-
Brief DescriptionAmphotericin B is a Lipid-based Polyene Antifungal and Polyene Antifungal. The chemical classification of amphotericin b is Polyenes.
-
DescriptionAmphotericin B is a Lipid-based Polyene Antifungal and Polyene Antifungal. The chemical classification of amphotericin b is Polyenes.(In Vitro):Amphotericin B administration is limited by infusion-related toxicity, including fever and chills, an effect postulated to result from proinflammatory cytokine production by innate immune cells. Amphotericin B induces signal transduction and inflammatory cytokine release from cells expressing TLR2 and CD14. Amphotericin B interacts with cholesterol, the major sterol of mammal membranes, thus limiting the usefulness of Amphotericin B due to its relatively high toxicity. Amphotericin B is dispersed as a pre-micellar or as a highly aggregated state in the subphase. Amphotericin B only kills unicellular Leishmania promastigotes (LPs) when aqueous pores permeable to small cations and anions are formed. Amphotericin B (0.1 mM) induces a polarization potential, indicating K+ leakage in KCl-loaded liposomes suspended in an iso-osmotic sucrose solution. Amphotericin B (0.05 mM) exhibits a nearly total collapse of the negative membrane potential, indicating Na+ entry into the cells.(In Vivo):Amphotericin B results in prolonging the incubation time and decreasing PrPSc accumulation in the hamster scrapie model. Amphotericin B markedly reduces PrPSc levels in mice with transmissible subacute spongiform encephalopathies (TSSE). Amphotericin B exerts a direct effect on Plasmodium falciparum and influences eryptosis of infected erythrocytes, parasitemia and hostsurvival in murine malaria. Amphotericin B tends to delay the increase of parasitemia and significantly delays host death plasmodium berghei-infected mice.
-
In Vitro——
-
In Vivo——
-
SynonymsAbelcet | AmBisome | Amphocin | Ampho-Moronal | Amphozone
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number1397-89-3
-
Formula Weight924.08
-
Molecular FormulaC47H73NO17
-
Purity>98% (HPLC)
-
SolubilityDMSO: 22 mg/mL (23.8 mM)
-
SMILESO=C([C@@H]([C@](O1)([H])C[C@@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)O3)[C@@H](O)C[C@@]1(O)C[C@@H](O)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)CC3=O)O
-
Chemical Name(1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-(((2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Sau K, et al. J Biol Chem, 2003, 278(39), 37561-37568.
molnova catalog



related products
-
Dihydroartemisinin
Dihydroartemisinin (DHA) is a semi-synthetic derivative of artemisinin and isolated from the traditional Chinese herb Artemisia annua.
-
2,2-Bipyridine
2,2'-Bipyridine is a unique bioactive natural product molecular scaffold commonly used in the core structure of many chelating ligands.
-
α-2,3-sialyltransfer...
α-2,3-sialyltransferase-IN-1 is a noncompetitive inhibitor of α-2,3-sialyltransferase [IC50: 6 μM].



 Cart
Cart
 sales@molnova.com
sales@molnova.com


